Summary
This research determined rates of deposition (removal by chemical reaction) of chlorine gas through Chemical Environmental Reactivity Tests (CERT) to determine the initial and maximum reactivity rate of chlorine with common environmental materials (bare soil and various common vegetation). This research recommended model parameters that can be used directly in atmospheric dispersion models for chlorine.
Background
In North America, chlorine is the second most transported toxic inhalation hazard gas. It is difficult for computer models to simulate chlorine releases because chlorine is denser than air. In 2010, the U.S. Department of Homeland Security Chemical Security Analysis Centre (US DHS CSAC) and the U.S. Defense Threat Reduction Agency launched the Jack Rabbit Research Program to close this knowledge gap.
The program was originally created to study small releases of chlorine and ammonia. Since 2013, Transport Canada’s (TC) Transportation of Dangerous Goods Directorate (TDG) has participated in the second phase of the Jack Rabbit Research Program (Jack Rabbit II) to better understand how large-scale chlorine releases behave in transportation scenarios.
With funding from Defence Research and Development Canada, TDG has participated in large-scale field trials (rail-car scale releases of chlorine) and a chlorine reactivity chamber study. The results of field trials were used to update the computer models that evaluate impact areas and validate the initial isolation and protective distances in the 2020 edition of the Emergency Response Guidebook (ERG) developed by TC, the US Department of Transportation and the Secretariat of Transport and Communications of Mexico, with help from the Centro de Informaciòn Quìmica para Emergencias (CIQUIME) of Argentina.
It is known that chlorine is very reactive with encountered environmental materials (namely plants) and the purpose of this study was to quantify the impact of chemical reactivity on chlorine plume behaviour. This reactivity is typically incorporated into dispersion models as dry deposition.
Previous studies have identified that there is a maximum reactivity limit, however this is the first study that has included cycling of the chlorine (creating a flow as opposed to injecting into a stirred closed container) as well as high chlorine concentrations (1000 ppm vs 50 ppm in previous Argonne Lab studies) which better represent a large release scenario. It is important to note that current atmospheric dispersion models do not account for this reactivity limit.
Objectives
The objective of the study included:
- development, design, and construction of a chamber capable of testing the reactivity of different materials when exposed to gases
- measure the reduction in chlorine concentration when exposed to environmental materials. The experiments were conducted for multiple wind speeds
- develop a model based on the measurements to calculate the reactivity rate and reactivity limit of each tested material which can be incorporated into dispersion models
Methods
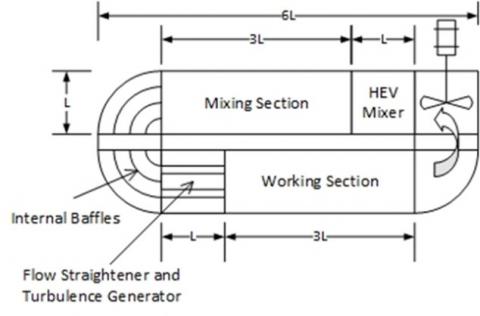
The CERT apparatus was designed to expose sample materials to chlorine under similar velocities and turbulence to that of the atmosphere.
Environmental materials inserted into the working section of the chamber are exposed to 1000 ppm of chlorine and the reduction in chlorine concentration (i.e. amount reacted) is measured for the duration of the experiment.
Multiple vegetations were chosen to represent a range of common environmental materials:
- white clover
- rye grass
- Norwegian spruce
- Jeffersred maple
- sugar maple
- bare soil
Results
The study determined the initial rate of deposition (i.e. removal by reaction) of chlorine for typical environmental surfaces as well as the maximum deposition representing the limit to the amount of chlorine that can react with the materials. In addition, a comparison was made between data results from this work to previous experimental results completed by Argonne National Laboratory. Both data sets were analyzed using the reaction models developed in this work to compare the dry deposition velocity (ks) and the maximum deposition (Mmax).
|
|
Reanalysis of Argonne National Laboratory Data |
This work |
||
|---|---|---|---|---|
|
Material |
ks” (cm/s) |
Mmax” (mg/m2) |
ks” (cm/s) |
Mmax” (mg/m2) |
|
White Clover |
0.30 |
700 |
0.0055 |
500 |
|
Shamrock |
0.077 |
5000 |
|
|
|
Rye Grass |
|
|
0.018 |
3,000 |
|
White Spruce |
0.12 |
5000 |
|
|
|
Norwegian Spruce |
|
|
0.017 to 0.0017 |
1000 |
|
Jeffersred Maple |
|
|
0.0015 |
2,000 |
|
Sugar Maple |
|
|
0.00032 |
2,000 |
|
Soil (0% moisture) |
0.54 |
5000 |
|
|
|
Soil (2% moisture) |
0.50 |
1000 |
|
|
|
Soil (4% moisture) |
0.57 |
600 |
|
|
|
Soil (8% moisture) |
0.71 |
600 |
|
|
|
Soil (this work) |
|
|
0.1 |
4,500 |
The Argonne National Laboratory data reflected significantly higher kinetic parameters. However, both data sets resulted in similar maximum deposition values when applied to the dispersion models. These results are being incorporated into the dispersion models used by Argonne National Laboratory which could result in updates to the distances in the ERG.
Conclusions
Analysis of the experimental data shows that the reaction between the chlorine and the environmental surfaces can be effectively modelled as first order surface reactions with a maximum limit (i.e., the rate of the reaction is proportional to the available surface exposed). Also, it was observed that fluid velocity and turbulence levels do not have a significant impact on reactivity of surfaces with chlorine.
In general, it was found that the reactivity of tree species were not as fast as other ground cover plants in this study. Though the effectiveness of ground cover plants could be limited if the plants are not active (i.e. Winter).
Future action
Testing of additional environmental materials such as additional plants and construction materials such as asphalt and concrete is ongoing. TC is contributing through participation on the US DHS CSAC project working group for this expanded research.
Acknowledgements
This project was conducted by Dr. Tom Spicer at the University of Arkansas with support from Sun McMaster and Dr. Shannon Fox at the U.S. Department of Homeland Security Science & Technology (DHS S&T) Chemical Security Analysis Center (CSAC), and Defence Research and Development Canada Centre for Security Science (DRDC CSS).
Report Title: Chlorine Reactivity with Environmental Materials in Atmospheric Dispersion Models
Authors: Tom Spicer, Shannon B. Fox
TP number: N/A
ISBN: N/A
Catalogue number: CSAC 20-011 (US Department of Homeland Security report number)
Contact
To obtain a copy of the report, please visit the U.S. Department of Homeland Security website.
If you have any questions, please contact us:
TDG Scientific Research Division
TC.TDGScientificResearch-RecherchescientifiqueTMD.TC@tc.gc.ca
Keywords
Chlorine releases, dispersion modeling, large scale release, reactivity