by Fred Scaffidi, Chemist
The behaviour of a chemical can be predicted by observing its physical properties. Our senses can detect a chemical as being solid or liquid, clear or coloured, odourless or pungent. In general however, physical properties are obtained using simple instruments such as a thermometer, a capillary tube and an electric heat source to measure the melting point; litmus paper to measure pH; and, a vacuum pump and glass apparatus to measure vapour pressure.
Vapour Pressure
In assessing hazards, it is very important to know the vapour pressure of a substance. The vapour pressure is the pressure at which a liquid and its vapour are in equilibrium at a given temperature. The vapour is said to be "pushing" against the atmosphere. In other words, the higher the vapour pressure the faster a liquid evaporates. When the vapour pressure reaches the atmospheric pressure, the liquid is at its boiling point. Vapour pressure is measured in units of atmospheres (atm), millimeters of mercury (mmHg) or kilopascals (kPa). As a point of reference, normal atmospheric pressure is 1 atm (760 mmHg or 101.325 kPa).
Let's look at some commonly used chemicals and their vapour pressures:
| Chemical | Class | Vapour Pressure at 20°C |
|---|---|---|
| Dichloromethane (used as a paint stripper) |
6.1 | 0.46 atm |
| Ethyl ether (Industrial Solvent) |
3.1 | 0.59 atm |
| Ethyl mercaptan (added to propane to give it an odour) |
3.1 | 0.59 atm |
| Methyl alcohol (Windshield washer fluid) |
3.2, 6.1 | 0.13 atm |
| Ethylene glycol (automobile antifreeze) |
NR* | 0.00006 atm |
| Toluene (used in aviation gasoline) |
3.2, 9.2 | 0.03 atm |
| Water | NR* | 0.02 atm |
-------
*(NR - non-regulated)
Spill Scenario
Using a typical spill scenario let's see what we can learn from the vapour pressure property.
The Product
Dichloromethane - UN 1593, Class 6.1
Organic solvent; clear liquid with a penetrating, ether-like odour.
Vapour pressure: 0.46 atm.
The Spill
Ten litres have spilled and resulted in a puddle with a surface area of approximately 2M2. (Note: Surface area is important since the larger the area, the more product can leave the surface; hence the shorter the evaporation time. Also, the higher its temperature, the faster a chemical will evaporate.)
Atmospheric Conditions
A slight 4 km/h wind and the temperature is 20°C.
Based on the above information, one would predict that dichloromethane would evaporate fairly quickly. This is indeed the case. It evaporates many times faster than water. Using computer modeling techniques, it was calculated that it would take 28 minutes for our 10 litre-puddle to evaporate to dryness under the conditions described above. A 10 litre-puddle of water could take hours to evaporate. A 10 litre-spill of ethyl mercaptan, a chemical with an extremely strong odour, would evaporate in 17 minutes. due to its low vapour pressure, ethyulene glycol takes a very long time to evaporate. this is one reason why ethylene glycol makes a good antifreeze.
Vapours represent the most insidious of hazards. Inhalation is a direct route into the human body. there has been much effort involved in setting standards for acceptable human exposure to airborne chemicals.
The American Conference of Governmental Industrial Hygienists (ACHIH) and others are very concerned with what type of breathing protection should be worn and when. The airborne concentration of a substance that a worker can be exposed to over a 8 hour day, 40 hour-work week is represented by a TLV-TWA value1. It is usually indicated in parts per million (ppm) or mg/cubic metre of air. The vapour pressure is an important factor when considering the TLV.
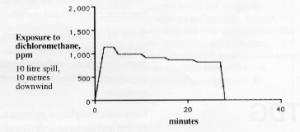
Going back to our computer model, someone standing 10 metres directly downwind from our spill would be exposed to approximately 1000 ppm of dichloromethane for the entire 28 minutes evaporation time as shown graphically below. The wind and temperature conditions are such that a fairly steady flow of dichloromethane vapour is generated. Thus this person would be at risk, since the dichloromethane TLV-TWA is 100ppm
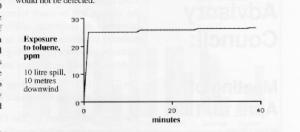
A similar graph for the toluene case shows that someone standing 10 metres downwind would be exposed to a constant level of approximately 27 ppm. The TLV-TWA for toluene is 100 ppm as well. Vapours would not represent as much of a hazard in the toluene case. If one were to keep upwind, vapours would not be detected.
These graphs are typical of the type of computer generated information available today and can help people assess the risks involved in dealing with a spill.
Incidentally, anyone living in a large city is constantly exposed to 0.5 ppm of toluene as a background level. This is primarily due to automobile exhaust!
The TLV-TWA* is generally used to describe situations involving long term or chronic exposure to small amounts of a chemical. It can also be used as an indicator of risk in an emergency. Massive short term or acute exposure to chemicals with the lowest toxicity can also represent a serious risk to the human body.
In the case described, exposure to 1000 ppm of dichloromethane would represent an acute exposure which could result in choking, followed by headache and possibly more serious consequences. On the other hand, no effects were observed in people who were exposed over several years to below 100 ppm.
_______________
* Treshold limit value - time weighted average.
References
- Dangerous Goods Initial Emergency Response Guide, 1992 Edition, Canada Communication Group - Publishing, Ottawa, Ontario, Catalogue Number T22-44/1992E
- Transport of Dangerous Goods Regulations, 1993, List II, Schedule II, Department of Supply and Services, Ottawa, Ontario, SOR 93/525
- NPIRI Raw Materials Data Handbook, Volume 1, National Printing Ink Research Institute, 19974, Bethlehem, Pennsylvania
- Hawley's Condensed Chemical Dictionary, 11th Edition, Sax and Lewis, published by Van Nostrand Reinhold Company, New York 1987, ISBN 0-442-28097-1
- Aerial Location of Hazardous Atmospheres Computer Model produced by the National Oceanic and Atmospheric Administration, Seattle, Washington, (The software is part of a larger package called CAMEO, Computer Aided Management of Emergency Operations.)
- The MSDS Pocket Dictionary, published by Genium Publishing Company, Schenectady, New York, 1990, ISBN 0-931690-27-7
- Environment Canada Manual for Spills of Hazardous Materials, published by Environment Canada Technical Services Branch, Ottawa, Ontario, 1984 ISBN 0-662-13186-X
- Environmental Health Criteria 52, United Nations Environment Program, World Health Organisation, Beneva, 1985
_______________
Publication: TDG Dangerous Goods Newsletter, Vol. 14, No. 1, Spring/Summer 1994.