by Eric LeBreton
A confined space may be defined as a place where the means of entry or exit are restricted because of location, design, construction or contents. It may include tanks, tankers, tunnels, silos, sewers, flues, pipelines, sea containers, vessels etc. The main hazards encountered in confined spaces are fire or explosion, asphyxiation, toxicity, drowning in liquids or free flowing solids and injury or death if mechanical equipment within the confined space is inadvertently turned on while someone is still inside. These hazards are due to the presence of hazardous gases, vapours, fumes, dusts or the creation of an oxygen-deficient or oxygen-rich atmosphere.
Fire and explosion
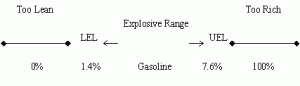
Fire or explosions may occur in confined spaces which have an atmosphere within the explosive limits of the gas (or liquid vapour). Explosive limits (also known as "flammable limits"), expressed in percentage (%), may be defined as the minimum and maximum concentrations of a flammable gas or vapour between which ignition can occur. Concentrations below the lower explosive limit ( LEL ) are too lean to burn while those above the upper explosive limit ( UEL ) are too rich.
Explosive / Flammable Limits
This may best be explained by using a gasoline engine as an analogy. If there is not enough gasoline vapours in the engine cylinders, the engine will not start (mixture too lean), which means that the LEL (gasoline: 1.4%) has not been reached. On the other hand, if the gasoline vapours are beyond the UEL (gasoline: 7.6 %), the engine will also not be able to start. This is commonly known as a "flooded engine" (mixture too rich).
It is important to note that the explosive limits are normally for gas or vapour / air mixtures. Mixtures in oxidizing gases will normally expand the explosive range (especially the UEL ) and have a bigger explosion effect. The gasoline engine analogy may be continued regarding the effect of adding nitrous oxide gas to the air intake of a race car engine. Nitrous oxide being an oxidizing gas, has the effect of providing an additional boost and increases the engine horsepower. Here are some examples of explosive limits of various chemicals in air and oxygen.
| Chemical - |
Explosive limit in air |
Explosive limit in oxygen |
|---|---|---|
| Ammonia | 15.50-27.00 | 13.50-79.00 |
| Carbon monoxide | 12.50-74.20 | 15.50-93.90 |
| Hydrogen | 4.00-74.20 | 4.65-93.90 |
| Methane | 5.00-15.00 | 5.40-59.20 |
| Diethyl ether | 1.85-36.50 | 2.10-82.00 |
| Propylene | 2.00-11.10 | 2.10-52.80 |
Dust particles have a minimum or lower explosive limit to almost no upper limit. Here are examples of minimum explosive limits (oz/ft3): Polystyrene (0.02) Cornstarch (0.04), Coal (0.055), Iron (0.12).
It is important to note an explosimeter gives a reading as a percentage. The reading is based on the percentage of the LEL and not the full concentration of the vapour or gas in the mixture. For example a 50% explosimeter reading of a gasoline/air mixture really translates into 0.7% gasoline (50% of 1.4%, the LEL ). It is common practice to consider a 10% explosimeter reading or 10% of the LEL , as a safe working area. When taking explosimeter readings, consider the possibility that the vapour or gas may have accumulated in recessed areas (top or bottom of tank depending on the density of the gas or vapour).
A liquid may not be considered flammable but may still have an explosive potential. An example is Dichloromethane or Methylene chloride UN1593 (often used in paint strippers) which evaporates very quickly. It is not flammable (no flash point) but its vapours may be explosive (explosive limits: 12 to 22 %). Dichloromethane may therefore not pose a fire hazard if spilled outdoors but it may have an explosive potential if spilled in a confined space. It would also be extremely dangerous to do any welding on a tank containing a residue of Dichloromethane even though this tank would not bear any flammable labels or placards.
A material may also not be regulated under TDG but may still pose an explosion hazard when heated. An example is Ethylene glycol (radiator antifreeze). It is not flammable at ambient temperature (flash point: 111°C) but it has explosive limits of 3.2 to 15.3%. A person may not enter a tank with hot material inside but may be asked to do some "hot work" (e.g. cutting, welding, drilling) which could potentially ignite the material inside the tank.
It is therefore pertinent to evaluate the explosion risk using proper monitoring equipment [e.g. explosimeter (vapours, gas), real-time aerosol monitor (dust particles)] to confirm that there is no explosive atmosphere before inspecting, entering, or working in a confined space.
Before working in a confined space, employees should follow proper grounding procedures and possibly wear anti-static garments. The human body can store up to 40 mJ of energy. The varpour/air mixture of many hydrocarbons can be ignited by just 1 mJ of energy. Some garments may create a potential exceeding 3000 Volts while being worn which may cause the ignition of gasoline/air mixtures.
In summary, to avoid explosions in a confined space, one must ensure he/ she is not carrying a static charge and that there is no ignition source present. An explosion-proof lighting system (no exposed light bulbs which may break) must be available. Explosion-proof fans may be used to lower the vapour concentrations to below the material's LEL but employees must ensure the contaminated air discharged from the confined space is not hazardous to workers outside. If ventilation alone can not remove the explosion hazard, it may be necessary to remove the material and clean the tank with solvents, detergents or steam. If a fine solid is involved and it does not react adversely with water, moistening with water may be helpful in cutting down on dust particles.
Gases
Most corrosive substances (acid, base, corrosive gases) give an indication of their presence by irritating the mucous membranes (the results being coughing, watering of the eyes etc.). However, many poisonous substances give little or no warning e.g. carbon monoxide (no odour, minor headache initially, prevents the assimilation of oxygen by the red blood cells), hydrogen sulphide (rotten egg odour disappears quickly due to olfactory fatigue, immediate collapse & respiratory paralysis at ~ 1800 ppm). Exposure to hydrocarbon solvents may cause headaches, nausea, dizziness, central nervous system depression and possibly death from pulmonary edema.
It is important to note that an employee may not always know the dangers he may encounter. For example, formic acid UN1779, which is a corrosive acid with a pungent odour, slowly decomposes to carbon monoxide and water. Drums containing formic acid are often fitted with a pressure relief device to vent any carbon monoxide formed. An employee may therefore not suspect an accumulation of carbon monoxide inside a storage room, marine container, tractor trailer, etc.
Asphyxiation due to lack of oxygen is probably the most dangerous risk associated with entering a confined space. To work in a confined space, regulatory agencies in Canada require a minimum oxygen concentration of between 18 and 19.5% and a maximum of 23%. Refer to your respective provincial or federal agency for the relevant oxygen standards. We breath 21 % oxygen at sea level. Oxygen concentrations for resting passengers in an aircraft are kept to a minimum of ~ 16%. Depending on physical exertion, oxygen concentrations of less than 16% may cause loss of judgment and affect muscular coordination. Between 10 and 12%, loss of consciousness may occur and below 6%, death occurs within minutes. An oxygen-deficient atmosphere may be detected in any confined space where oxygen has been consumed by a chemical reaction (e.g. rust) or displaced by another gas (e.g. nitrogen). The oxygen concentration in a slightly rusted tank with traces of salt and in high humid conditions, may go down to next to nothing in 24 hours or less. Therefore always take an oxygen reading before entering a tank even though a reading had been taken a few hours beforehand. The materials in many tanks are blanketed with nitrogen gas. A person opening the tank hatch could lose consciousness due to lack of oxygen and fall inside the tank.
The main concern with oxygen concentrations above 23% is the possibility that materials which would normally not ignite at normal oxygen levels, would be prone to combustion. Oxygen-rich atmospheres may be found in tanks that previously contained oxidizing compounds (e.g. hydrogen peroxide, sodium chlorate etc. ) which have decomposed, often when heated, to release oxygen.
In ventilating a confined space, it is important to consider what will consume the oxygen (how many employees inside, welding work etc.) and if a forced exhaust fan is necessary to remove contaminants (e.g. welding fumes).
Other cases
Here are some examples of confined spaces where no dangerous goods are stored but hazards exist:
- Sewers: Decomposing organic matter, such as domestic waste or plant life, may produce flammable gases (methane), flammable and toxic gases (hydrogen sulphide), asphyxiant gases (carbon dioxide) and an oxygen-deficient atmosphere due to rust or bacterial respiration. Defective wiring in a wet atmosphere may electrocute an employee.
- Hay/Grain Silos: Dust explosions are well known to occur in grain silos. Grain may also agglomerate and form a bridge or dome across a bin. This grain may fall and entrap or suffocate a grain worker. An employee may be injured while working inside a silo if an auger or other piece of equipment is turned on. Fermentation of corn, wheat, potatoes etc. may give off large quantities of carbon dioxide gas (asphyxiant) and ethyl alcohol (flammable). Toxic nitrogen dioxide (NO2) gas is also formed when nitric oxide in fresh silage comes in contact with oxygen. Brief exposures (<10 150 minutes) to ~ ppm of NO2 may cause coughing, headache, nausea, vomiting etc. This sickness is known as "silo-filler's disease". The grain in the silo also have been fumigated with a fumigant (e.g. aluminum phosphide) which slowly releases toxic and flammable phosphine gas under humid conditions. < li>
General guidelines to follow before entering a confined space
- A permit, pre-authorized entry plan, written records of steps followed and approved training may be required
- Ensure safe access and egress.
- Electrical power and piping for hazardous material must be locked shut.
- Use approved calibrated instruments to test and continually monitor flammable atmospheres and oxygen content.
- Ensure proper removal of contaminants and adequate air exchange using ventilation and/or exhaust fans.
- If adequate oxygen or a safe atmosphere cannot be guaranteed, approved breathing apparatus and protective clothing must be worn.
- The person entering the confined space should have an attached approved safety harness.
- Properly trained people should be stationed outside the confined space in communication with the person inside the confined space and able to aid in emergency removal and medical care (cardiopulmonary resuscitation).
- Access to first aid kits, water (eye wash, shower) and rescue equipment (stretcher, hoist) must be assured.
- Immediate access to communication equipment to report incidents and request medical aid must be available.
_______________
Publication: TDG Dangerous Goods Newsletter, Vol. 16, No. 1, Spring 1996.