This document does not change, create, amend or suggest deviations to the Transportation of Dangerous Goods Regulations (TDG Regulations).
On this page
- Infectious substances
- Training
- Classification
- Class 6.2 substances subject to the TDG Regulations
- Samples versus cultures
- Doctor-patient confidentiality
- Assistance to classify infectious substances
- Means of containment
- Type P620 packaging
- Identify a Type P620 packaging
- Type P650 packaging
- Identify a Type P650 packaging
- Types of standardized and non-standardized packagings to transport clinical, (bio) medical or regulated medical waste
- Packagings permitted for clinical, (bio) medical or regulated medical waste assigned to UN2814 or UN2900
- Packagings permitted for clinical, (bio) medical or regulated medical waste assigned to UN3291
- Packagings permitted for solid medical waste assigned to UN3549
- To buy packagings for the transportation of infectious substances intended for disposal or waste
- Documentation
- Labels and placards
- Exemptions and special provisions
- Marine shipments
- Air shipments
- Quick reference guide – Road transport
- Compliance with the Transportation of Dangerous Goods Act and Regulations
- Contact us
- Appendix – Transporting Ebola contaminated waste
Infectious substances
An infectious substance, as defined under Section 1.4 of the TDG Regulations, is a substance known or reasonably believed to contain viable micro-organisms, such as viruses, bacteria, parasites, fungi, and other agents such as prions, that are known or reasonably believed to cause disease in humans or animals. Substances classified as infectious substances may include blood, tissue, organs, body fluids or materials contaminated by them, or cultures, that contain or may contain pathogenic.
A list of pathogens, such as Ebola virus or Herpes B virus, classified by category, can be found in Part 2 of the TDG Regulations, under Appendix 3.
Note: The table under Appendix 3 is not a complete list. If a pathogen is not listed in Appendix 3, it is still considered an infectious substance when it meets the definition above and exhibits characteristics similar to a pathogen on the list.
In addition to the TDG Regulations, other government departments regulate infectious substances. The Public Health Agency of Canada (PHAC) administers regulations that apply to human pathogens in Canada, while the Canadian Food Inspection Agency (CFIA) regulates animal pathogens. In addition, provincial governments may have additional regulations that pertain to infectious substances.
As a consignor, you must comply with the TDG Regulations requirements such as:
- Classification (Part 2 – TDG Regulations)
- Documentation (Part 3 – TDG Regulations)
- Dangerous Goods Safety Marks (Part 4 – TDG Regulations)
- Means of Containment (Part 5 – TDG Regulations)
- Training (Part 6 – TDG Regulations)
- Emergency Response Assistance Plan (ERAP) (Part 7 – TDG Regulations)
- Reporting Requirements (Part 8 – TDG Regulations)
Training
Always assume training is required, unless you are using an exemption which exempts you from Part 6 of the TDG Regulations. You will find most exemptions in Part 1 of the TDG Regulations, from Sections 1.15 to 1.50. More specifically, exemptions related to infectious substances can be found at Sections 1.39 and 1.42.3.
Employers are responsible for issuing a training certificate once their employee has received adequate training. The certificate must contain all of the information required by Section 6.3 of the TDG Regulations. Even though there is no standard format, the TDG Directorate provides a sample in the TDG Bulletin – TDG Training.
Classification
Infectious substances are classified as Class 6.2, Infectious Substances, and are assigned to a category (Category A or Category B) instead of a packing group.
Category A
Infectious substances included in Category A are infectious substance that are transported in a form such that, when it is released outside of its means of containment and there is physical contact with humans or animals, it is capable of causing permanent disability or life-threatening or fatal disease to humans or animals. The proper shipping name of a Category A infectious substance is, as appropriate:
- UN2814 – INFECTIOUS SUBSTANCE, AFFECTING HUMANS
- UN2900 – INFECTIOUS SUBSTANCE, AFFECTING ANIMALS only
Category B
Infectious substance included in Category B are infectious substance for which the likely consequences of an exposure will not cause permanent disability and will not lead to fatality. The proper shipping name of a Category B infectious substance is:
- UN3373 – BIOLOGICAL SUBSTANCE, CATEGORY B
Classification of patient specimens
The following table is a guide for classification of infectious substances contained in patient specimens.
| Patient Specimen |
There is no reason to believe, based on sufficient information, the sample contains infectious substances |
There is a reason to believe that, based on the known medical history, the symptoms, the individual circumstances, or the endemic local conditions, the sample contains or may contain an infectious substance. |
Category A infectious substance |
Category B infectious substance |
|---|---|---|---|---|
| Classification |
“Exempt Human Specimen” or “Exempt Animal Specimen” |
UN2814 UN2900 UN3373 UN3291 |
UN2814 UN2900 |
UN3373 |
| Exemption |
See Section 1.42, Human or Animal Specimens Believed Not to Contain Infectious Substances Exemption, of the TDG Regulations |
See Section 2.36(2) and (3) |
See Section 1.39 of the TDG Regulations |
No reason to believe
The term “no reason to believe” means that sufficient information is available and none of it suggests that the specimens could contain infectious substances included in Category A or B.
Professional judgment is required to determine whether there is no reason to believe a specimen contains an infectious substance. Factors such as the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions should be considered.
For more details regarding the classification of infectious substances contained in patient specimens, please consult the document on classification of patient specimens.
Class 6.2 substances subject to the TDG Regulations
Any substance known or believed to contain infectious substances that meets the criteria of Category A or Category B is regulated under the TDG Regulations and must be assigned to UN2814, UN2900, UN3373, UN3291, or UN3549 as appropriate.
A list of regulated infectious substances can be found in Appendix 3 of Part 2 of the TDG Regulations. Note that this list is not exhaustive, but rather serves as a guide to classify pathogens.
Medical or clinical waste
Medical or clinical waste includes sharp objects, sampling materials, samples for disposal, soiled linen, etc. They are derived from the medical treatment of humans, the veterinary treatment of animals or from bio-research. They can be assigned to:
- UN2814 – INFECTIOUS SUBSTANCE, AFFECTING HUMANS, if they contain Category A infectious substances
- UN2900 – INFECTIOUS SUBSTANCE, AFFECTING ANIMALS only, if they contain Category A infectious substances affecting animals only
- UN3291 – CLINICAL WASTE, UNSPECIFIED, N.O.S.; (BIO) MEDICAL WASTE, N.O.S.; or REGULATED MEDICAL WASTE, N.O.S., if:
- the medical or clinical waste contains Category B infectious substances
- the medical or clinical waste are reasonably believed to have a low probability of containing infectious substances
- UN3549 – MEDICAL WASTE, CATEGORY A, AFFECTING HUMANS, solid or MEDICAL WASTE, CATEGORY A, AFFECTING ANIMALS only, solid
- Note: UN3549 is for solid medical waste containing Category A infectious substances generated from the medical treatment of humans or veterinary treatment of animals; it cannot be used for waste from bio-research or liquid waste.
See the Appendix for more information on transporting Ebola contaminated waste, as an example.
Biological products
Biological products are derived from living organisms and are used to prevent, treat or diagnose disease in humans or animals. They are also used for development, experiment or investigation purposes and include finished or unfinished products, live vaccines or attenuated live vaccines. It is important to note “Biological Products” and “Biological Substances” are not synonyms under the TDG Regulations.
A biological product known or reasonably believed to contain a pathogen that meets the definition of a Category A or B infectious substance must be assigned to UN2814, UN2900, or UN3373, as appropriate.
Genetically modified microorganism and organisms (GMMO and GMO)
GMMOs and GMOs which do not meet the definition of infectious substances are not subject to the TDG Regulations unless they meet the criteria for inclusion in another class.
Patient specimens
Patient specimens are those collected directly from humans or animals and include, for example, excreta, blood and its components, tissue and body fluids samples, being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention.
Samples versus cultures
Infectious substances can be transported as samples, including patient specimens, or cultures (the result of a process by which pathogens are intentionally propagated). The risk of infection is higher in cultures, due to the high concentration of pathogens as opposed to patient specimens.
Under certain conditions, infectious substances classified as Category A and contained in samples may be shipped as Category B.
However, some Category A infectious substances must always be shipped as Category A regardless of their form due to their pathogenicity. The following table provides the list of infectious substances that must always be shipped as Category A. See Subsection 2.36(2) and (3).
| Name of infectious substance | UN number |
|---|---|
|
UN 2814 |
Human or animal specimens are exempted from certain parts of the TDG Regulations if there is no reason to believe, based on sufficient information, that the specimen contains an infectious substance. You can ship such specimens using the exemption under Section 1.42 of the TDG Regulations.
Patient specimens may be shipped as per Section 1.42 of the TDG Regulations if a professional judgment concludes there is no reason to believe the patient could be contaminated with an infectious substance, even if the samples are collected for the purpose of testing for a known infectious substance. For example, an employer may wish to screen all new employees for infectious diseases. In this case, you may ship the sample as “Exempt Human Specimen” if the medical professional concludes there is no reason to believe that the person has been in contact with an infectious substance or has symptoms associated with an infectious disease.
Professional judgment must be based on factors such as the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions.
Note: If other dangerous goods are transported with exempt patient specimens (dry ice, formalin, etc.), the regulations will apply, unless it meets another exemption.
Doctor-patient confidentiality
There are no exemptions that apply to shipping samples that are known or suspected to contain infectious substances. However, the TDG Regulations do not require you to include a patient’s name or any personal reference when shipping infectious substances.
Shipping known or suspected infectious substances without complying with the TDG Regulations is an offence for which enforcement actions are applicable.
Assistance to classify infectious substances
The TDG Directorate is the authority with regards to the classification of infectious substances for transportation. However, you can contact the Public Health Agency of Canada (PHAC) or the Canadian Food Inspection Agency (CFIA) for assistance in the classification of infectious substances.
Public Health Agency of Canada
Phone: (613) 957-1779
E-mail: PHAC.pathogens.pathogenes.ASPC@canada.ca
Canadian Food Inspection Agency
Phone: (613) 773-5327
E-mail: biocon@inspection.gc.ca
Means of containment
The following types of packaging are required to ship infectious substances:
- Type P620
- Type P650
- standardized and non-standardized packagings permitted in Part III of the CAN/CGSB 43.125 standard for the transport of infectious substances intended for disposal or clinical, (bio) medical or regulated waste
A copy of the CAN/CGSB-43.125 Standard can be obtained from Public Service and Procurement Canada.
| Type of packaging | UN number – category |
|---|---|
|
Type P620 |
Intended for transport of:
May also be used for transport of:
|
|
Type P650 |
Intended for transport of:
May also be used for transport of:
|
|
Standardized and non-standardized |
Intended for transport of:
Note: Permitted packaging is dependent on classification of waste |
Type P620 packaging
It is defined as a packaging that complies with the requirements of the CAN/CGSB-43.125 standard for Type P620 packaging or, if it is manufactured outside Canada, complies with the requirements of Chapter 6.3 and Packing Instruction P620 of the UN Recommendations and the national regulations of the country of manufacture.
This packaging is intended to transport an infectious substance of Category A. As it is the highest integrity packaging, this packaging can also be used to transport Category B infectious substances and clinical, (bio) medical or regulated waste.
You will find the requirements for the design, testing and marking of Type P620 packaging in Part I of the CAN/CGSB-43.125 standard. Facilities that manufacture Type P620 packaging in Canada must be registered with Transport Canada and must have their packaging design registered with Transport Canada. The CAN/CGSB-43.125 standard now requires the periodic retest of a Type P620 packaging design every five years.
Identify a Type P620 packaging
A Type P620 packaging will have a UN marking on the outer packaging as set out in Section 5.1 of the CAN/CGSB-43.125 standard. For example:
4G/CLASS 6.2/21
CAN/ABC 8-9999
Description of the UN packaging code and symbol
A Type P620 packaging shall consist of:
- inner packagings comprising:
- leakproof primary receptacle(s)
- leakproof secondary inner packaging(s)
- a rigid outer packaging of adequate strength for its capacity, mass and intended use of which the smallest external dimension is at least 100 mm. The outer packaging shall be selected from Table 1 of the CAN/CGSB-43.125 standard
For liquid infectious substances, an absorbent material must be placed between the primary receptacle(s) and the secondary inner packaging and in sufficient quantity to absorb the entire content of the primary receptacle(s).
The primary receptacle(s) of a Type P620 packaging cannot be reused. The secondary inner packaging or outer packaging of a Type P620 packaging may be reused if there is no visible contamination, damage or defects that may render the packaging unsafe for transport.
Example of a Type P620 packaging:
Source: IATA, Montreal, Canada (modified by the TDG Directorate)
Text version
Type P620 packaging, which is a triple packaging system.
The outer packaging is a UN specification box, with UN specification marking. The box is also marked with a Class 6.2 infectious substance label. Next to label is the shipping name "infectious substance affecting humans" and the UN number. There are also labels for the consignor (i.e. shipper) and consignee and a label for the responsible person that includes their telephone number. Finally, the outer packaging is labelled with an orientation label, which is two arrows pointing up. These arrows are only required for Air and Marine transport when the primary receptacle exceeds 50 ml or 50 g.
Placed inside the outer package is a secondary packaging, which is leakproof. This secondary packaging is cylindrical in shape and has a screw-on cap. The secondary packaging is affixed with a label that includes an itemized list of its contents.
Finally, inside the secondary packaging is the leakproof primary receptacle, which is a test tube. The test tube is surrounded by absorbent material.
Note: Though often pre-printed on the box, orientation arrows and the name of a responsible person are not required for road transportation.
Type P650 packaging
A Type P650 packaging is defined as a packaging that complies with the requirements of the CAN/CGSB-43.125 standard for Type P650 packaging or, if it is manufactured outside Canada, complies with the requirements of Packing Instruction P650 of the UN Recommendations and the national regulations of the country of manufacture.
A Type P650 packaging is intended for the transport of UN3373, BIOLOGICAL SUBSTANCE, CATEGORY B. This packaging can also be used to transport clinical, (bio) medical or regulated waste classified as UN3291.
You will find the requirements for the design, testing and marking of Type P650 packaging in the CAN/CGSB-43.125 standard.
Facilities that manufacture Type P650 packaging in Canada are not required to be registered with Transport Canada. However, a Type P650 packaging design report must be prepared and retained by the manufacturer in accordance with Annex A of the Standard to demonstrate compliance with the Standard.
Identify a Type P650 packaging
The marking required on a Type P650 packaging is a hybrid marking as it is used as the packaging mark as specified in Section 5.2 of the CAN/CGSB-43.125 standard and the dangerous goods mark, (Category B mark) found in the Appendix of Part 4 of the TDG Regulations.
The Category B mark, as per Section 4.22.1 of the TDG Regulations, must be displayed on the outside of a Type P650 packaging to demonstrate compliance with the CAN/CGSB-43.125 Standard.
The marking shall be in the form of a square on point with each side having a length of at least 50 mm. The width of the line shall be at least 2 mm and the letters and numbers shall be at least 6 mm high. The illustration below shows the marking for Type P650 packaging.
Special Provision 165 of the TDG Regulations allows the use of this mark even if the packaging is empty. As such, having an empty packaging with this mark displayed is not considered misleading dangerous goods mark.
A Type P650 packaging shall consist of:
- inner packagings comprising:
- primary receptacle(s) (leakproof or siftproof)
- secondary packaging(s) (leakproof or siftproof)
- an outer packaging of which the smallest external dimension is at least 100 mm
Note: Either the secondary packaging(s) or the outer packaging shall be rigid.
When transporting liquid infectious substances, absorbent material must be placed between the primary receptacle(s) and the secondary packaging in sufficient quantity to absorb the entire contents of the primary receptacle(s) so that any release of the liquid substance will not compromise the integrity of the cushioning material or of the outer packaging.
When transporting solid infectious substances, if there is any doubt as to whether any residual liquid may be present in the primary receptacle during transport, then a packaging suitable for liquids, including absorbent materials, shall be used.
The primary receptacle(s) of a Type P650 packaging cannot be reused. The secondary inner packaging or outer packaging of a Type P650 packaging may be reused if there is no visible contamination, damage or defects that may render the packaging unsafe for transport.
Examples of a Type P650 packaging:
Source: IATA, Montreal, Canada (modified by the TDG Directorate)
Text version
Type P650 packaging.
The outer packaging is a box that is labelled with the Category B mark, which is a square on point mark with "UN3373" written inside. Next to label is the shipping name "Biological Substances Category B". Also written on the box is the 24 hour telephone number. There are also labels for the consignor (i.e. shipper) and consignee.
Placed inside the outer package is a secondary packaging, which is leakproof or siftproof. This secondary packaging is cylindrical in shape and has a screw-on cap. The secondary packaging is affixed with a label that includes an itemized list of its contents.
Inside the secondary receptacle is a rack-type holder made from styrofoam or sponge. This rack is holding the primary receptacles, which are test tubes and surrounded by cushioning and absorbent material.
Types of standardized and non-standardized packagings to transport clinical, (bio) medical or regulated medical waste
There are various options of standardized and non-standardized packagings permitted in Part III of the CAN/CGSB-43.125 standard for the transport of clinical, (bio) medical or regulated medical waste. The packaging options are dependent on the classification of the waste. The packaging options are summarized in the table below with a more comprehensive description following the summary table.
| Classification of clinical, (bio) medical or regulated medical waste |
Permitted packaging |
|---|---|
|
UN2814 UN2900 |
|
|
UN3291 |
|
|
UN3549 |
|
Example of triple-layer packaging:
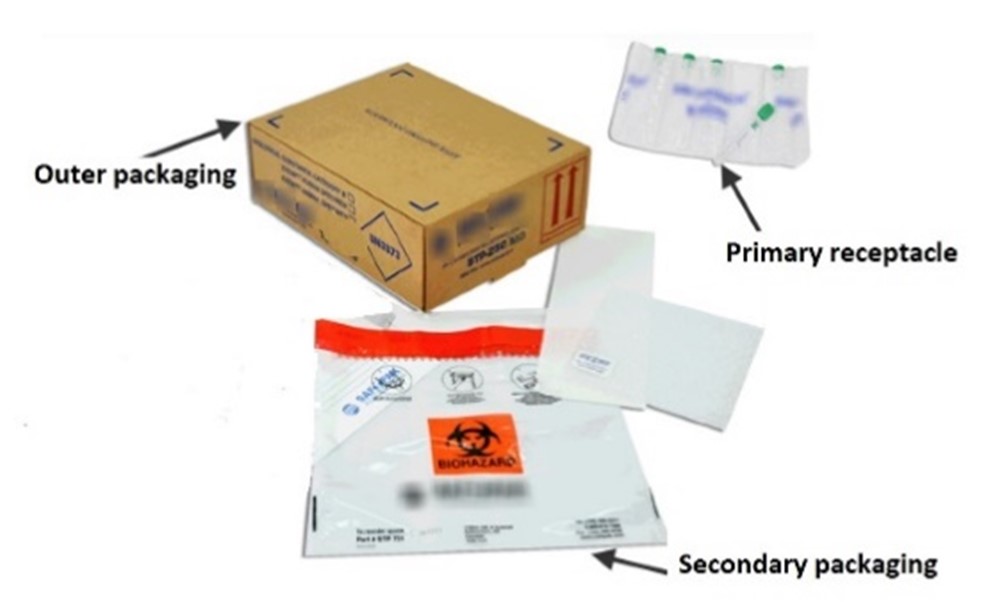
Text version
Example of a triple layer packaging with all dangerous goods marks. Box as outer packaging, plastic bas as secondary packaging and primary receptacle in plastic as inner packaging.
Packagings permitted for clinical, (bio) medical or regulated medical waste assigned to UN2814 or UN2900
A Type P620 packaging is permitted. Consult the Type P620 packaging section to learn more.
Packagings permitted for clinical, (bio) medical or regulated medical waste assigned to UN3291
UN standardized small container
The UN standardized small container must be a drum, jerrican, box, or composite packaging listed in Table 3 of the CAN/CGSB-43.125 standard and must meet a Packing Group I or II performance level.
The packaging must be leakproof or be made leakproof by inserting a plastic bag in the packaging. The plastic bag must pass the Elmendorf tear strength and the Dart impact strength tests as specified in Table 6 of the CAN/CGSB-43.125 standard. The packaging will bear a UN compliance marking.
UN standardized intermediate bulk container (IBC)
The UN standardized IBC must be listed in Table 4 of the CAN/CGSB-43.125 standard and must meet a Packing Group I or II performance level. The packaging will bear a UN compliance marking.
UN standardized large packaging
The UN standardized large packaging must be listed in Table 5 of the CAN/CGSB-43.125 standard and must meet a Packing Group II performance level. The packaging will bear a UN compliance marking.
Non-standardized combination packaging
This type of package consists of a securely closed plastic film bag placed inside either a:
- packaging that is rigid, leak-proof and designed for repeated use
- inside a fibreboard box that meets the capacity and performance requirements listed in Table 7 of the CAN/CGSB-43.125 standard
There is no packaging compliance mark required on this packaging.
Note: The plastic bag must pass the Elmendorf tear strength and the Dart impact strength tests as specified in Table 6 of the CAN/CGSB-43.125 standard.
A Type P620 packaging
Consult the Type P620 packaging section to learn more.
A Type P650 packaging
Consult the Type P650 packaging section to learn more.
Packaging for sharp objects
A packaging intended to contain sharp objects (for example, broken glass and needles) must meet the requirements of the CAN/CSA-Z316.6 standard or be rigid, leakproof, puncture resistant and designed for repeated use.
Example of a sharps container:
Text version
Picture of a red rigid plastic container with clear lid. A label indicating the presence of regulated medical waste with the biohazard symbol is displayed one side of the container.
Packagings permitted for solid medical waste assigned to UN3549
The packaging is a triple layer packaging consisting of:
- metal or plastic inner packaging
- metal or plastic intermediate packaging
- a UN standardized packaging of code 1A2, 1B2, 1D, 1G, 1H2, 1N2, 3A2, 3B2, 3H2, 4A, 4B, 4D, 4G, 4H2 or 4N or a code 50A, 50B, 50N, 50D, 50G, 50H meeting the packaging group II performance level for solids, at minimum
A fiberboard box meeting certain specifications may be used as an outer packaging in place of the UN packaging codes listed.
The packaging requirements for transport of UN3549 were introduced into the 2021 edition of the CAN/CGSB-43.125 standard. A person transporting this waste must follow the packaging requirements prescribed within the standard.
To buy packagings for the transportation of infectious substances intended for disposal or waste
The Transport Canada website provides a list of vendors for Type P620 and P650 packaging.
Transport Canada does not have a list of vendors for packagings permitted in Part III of the CAN/CGSB-43.125 standard because many different types of standardized and non-standardized packagings are permitted. You must ensure that your shipment meets the requirements listed in the standard for these types of packaging.
To learn more about Type P620, Type P650 and packagings permitted for the transportation of clinical, (bio) medical or regulated waste, you can:
- read the TDG FAQ on Packaging for Infectious Substances
- email one of TDG engineers at: tdgcontainers-tmdcontenants@tc.gc.ca
Documentation
Shipping document
You must prepare a shipping document if you are shipping a Category A infectious substance (UN2814 or UN2900). However, you will not need a shipping document if you are shipping as infectious substances classified as Category B (UN3373) in accordance with the exemption set out in Section 1.39 of the TDG Regulations.
Don’t forget that there are certain Category A infectious substances that can be shipped as a Category B. You need to refer to Subsections 2.36(2) and (3) of the TDG Regulations to verify which Category A infectious substances can be shipped as a Category B.
To learn more, or to view a sample shipping document, consult the TDG Bulletin – Shipping Documents.
Labels and placards
The dangerous goods marks that must be displayed on a small means of containment depend on the type of infectious substance you are shipping.
Category A
If you are shipping a Category A, you must display the infectious substance label on the package. This label is illustrated in the Appendix to Part 4 of the TDG Regulations.
Infectious substance label - Text version
Class 6.2 infectious substance label, which is a diamond shaped mark. This label has the biomedical symbol at the top and the number 6 at the bottom. In the center of the label there is bilingual text that reads as follows:
Infectious in case of damage or leakage immediately notify local authorities and CANUTEC 613-996-6666
Infectieux en cas de dommage ou de fuite communiquer immédiatement avec les autorités locales et CANUTEC 613-996-6666
The text on the label is:
INFECTIOUS
IN CASE OF DAMAGE
OR LEAKAGE
IMMEDIATELY
NOTIFY
LOCAL AUTHORITIES
AND
INFECTIEUX
EN CAS DE DOMMAGE
ON DE FUITE
COMMUNIQUER
IMMEDIATEMENT
AVEC LES AUTORITÉS
ET
CANUTEC
613-996-6666
Extra marking requirements:
The shipping name and UN number:
- UN2814 – INFECTIOUS SUBSTANCE, AFFECTING HUMANS
- UN2900 – INFECTIOUS SUBSTANCE, AFFECTING ANIMALS only
(No technical name (SP16))
Category B
When shipping a Category B infectious substance, you must display the Category B Mark on the package as per Section 1.39 and Section 4.22.1 and as illustrated in the appendix to Part 4 of the TDG Regulations.
Category B mark - Text version
Category B Mark. Square on a point. White background with UN3373 in the centre.
The text on the mark is:
UN3373
Extra marking requirements:
The shipping name:
- UN3373, BIOLOGICAL SUBSTANCE, CATEGORY B
Text on the package is:
- 24-Hour Number: 999-999-9999
Placards on the vehicle
As per Subsection 4.15(1) of the TDG Regulations, placards are required in the following instances:
- The infectious substances are transported in a large means of containment. By definition, a large means of containment is a means of containment with a capacity greater than 450 L. However, Section 4.16.1 provides a placarding exemption for dangerous goods having a gross mass of 500 kg or less.
- Paragraph 7.2(1)(g) of the TDG Regulations lists 16 infectious substances that require an Emergency Response Assistance Plan (ERAP). For those situations, the placards and UN number must be displayed. The placarding exemption found in Section 4.16.1 cannot be used when an ERAP is required.
- The person who loads the vehicle or large means of containment is responsible for displaying the placards. This person could be either the consignor (i.e., shipper) or the carrier. Once the vehicle leaves the site, the carrier is responsible for placarding.
Note 1: Placarding requirements apply to the person transporting the infectious substance or to the person loading the vehicle or large means of containment.
Note 2: The primary class placard must be displayed when infectious substances are transported unless the placarding exemption is used and no ERAP is required (Section 4.16.1)
Note 3: Placards and UN number are required when the shipment is transported in a large means of containment, and it requires an ERAP in accordance with Part 7 of the TDG Regulations.
Exemptions and special provisions
There are two exemptions for shipping Category B infectious substances or potential infectious substances. Like most exemptions, you can find them in Part 1 of the TDG Regulations.
- Section 1.39 – Class 6.2, Infectious Substances, UN3373, BIOLOGICAL SUBSTANCE, CATEGORY B exemption
- Section 1.42.3 – Medical or Clinical Waste
In order to use an exemption, you must comply with all conditions listed in the exemption. If you can’t, then you need to ship your infectious substances fully regulated.
Special provisions that only apply to infectious substances
Special Provision 84 states that an approved Emergency Response Assistance Plan (ERAP) is required for the dangerous goods referred to in paragraph 7.2(1)(g) (any quantity of dangerous goods that are Risk Group 4 human pathogens within the meaning of the Human Pathogens and Toxins Act).
Special Provision 128 exempts decontaminated medical or clinical waste from all parts of the TDG Regulations (except Parts 1 and 2) under certain conditions.
Special Provision 164 allows for the transport of other dangerous goods in the same small means of containment with UN2814, UN2900 or UN3373, if they are necessary for maintaining the viability or stability of the infectious substances, or for preventing their degradation.
Special Provision 165 of the TDG Regulations allows the use of the CATEGORY B mark even if the packaging is empty.
Marine shipments
When shipping by vessel, you must refer to Part 11 of the TDG Regulations.
Air shipments
When shipping by air, you must refer to Part 12 of the TDG Regulations.
For liquid infectious substances transported by aircraft, Type P650 packages must undergo the internal pressure test in accordance with Section 7.5 of the CAN/CGSB-43.125 standard.
Domestic transport
When transporting infectious substances domestically by air, Part 12 of the TDG Regulations requires you to comply with the ICAO Technical Instructions and Subsection 12.1(1) of the TDG Regulations.
International transport
When transporting infectious substances internationally by air (from or to Canada), Part 12 of the TDG Regulations requires you to comply with the ICAO Technical Instructions and Subsection 12.1(1) of the TDG Regulations.
Quick reference guide – Road transport
| Item | Category A | Category B | Waste |
|---|---|---|---|
| Classification |
UN2814 INFECTIOUS SUBSTANCE, AFFECTING HUMANS UN2900 INFECTIOUS SUBSTANCE, AFFECTING ANIMALS only |
UN3373 BIOLOGICAL SUBSTANCE, CATEGORY B |
UN2814 or UN2900 if waste contains Category A UN3291 if waste contains Category B or if the shipper has reasonable grounds to believe that there is a low probability of containing infectious substances UN3549 MEDICAL WASTE, CATEGORY A, AFFECTING HUMANS, solid or MEDICAL WASTE, CATEGORY A, AFFECTING ANIMALS only, solid |
| Packaging selection |
Type P620 |
Type P620 Type P650 |
Type P620 Type P650 A standardized or non-standardized packaging permitted in Part III of CAN/CGSB-43.125 standard |
| Documentation | Yes | Yes, unless shipped in accordance with Section 1.39 of the TDG Regulations | Yes, unless shipped in accordance with Section 1.42.3 of the TDG Regulations |
| Dangerous Goods Marks |
Yes, Class 6.2 label Shipping name and UN number (no technical name) |
Yes | Yes, unless shipped in accordance with Section 1.42.3 of the TDG Regulations |
| Placards |
Yes, if:
No, if:
|
Yes, unless shipped in accordance with Section 1.39 of the TDG Regulations | Yes, unless total gross mass of shipment is 500 kg or less |
| Training | Yes | Yes | Yes, unless shipped in accordance with Section 1.42.3 of the TDG Regulations |
Compliance with the Transportation of Dangerous Goods Act and Regulations
Failure to comply with the TDG Act and TDG Regulations may lead to fines and/or imprisonment. For more information, you can visit the TDG website and the Department of Justice website.
Contact us
For regulatory questions, contact the TDG regional office in your region:
Atlantic
1-866-814-1477
TDG-TMDAtlantic@tc.gc.ca
Quebec
1-514-633-3400
TMD-TDG.Quebec@tc.gc.ca
Ontario
1-416-973-1868
TDG-TMDOntario@tc.gc.ca
Prairie & Northern
1-888-463-0521
pnrtdg-tmdrpn@tc.gc.ca
Pacific
1-604-666-2955
TDGPacific-TMDPacifique@tc.gc.ca
Purchase of publications
- International Civil Aviation Organization (ICAO) – Air
- International Maritime Dangerous Goods Code (IMDG Code) – Ship / Vessel
Appendix – Transporting Ebola contaminated waste
Transport Canada regulates the Ebola virus as an infectious substance under the TDG Regulations. Following the Ebola Outbreak of 2014-2016, a new UN number for solid infectious substance waste of Category A (UN3549), as well as two associated packing instructions (P622 and LP622) were introduced into the 21st edition of the UN Model Regulations.
Prior to this, there were no approved packagings suitable for transporting the large volumes of Category A waste that are generated from caring for a patient suspected or known to be infected with the Ebola virus. Transport Canada will be introducing the UN3549 classification into the TDG Regulations. The packaging requirements for UN3549 were introduced into the 2021 edition of the CAN/CGSB 43.125 standard.
Anyone handling, offering for transport or transporting this infectious substance by road, rail, marine or air must comply with the TDG Regulations:
- Part 3 requires the consignment to be accompanied by a shipping document
- Part 4 requires the means of containment to display the appropriate dangerous goods marks
- Part 5 and the requirements listed in the CAN/CGSB 43.125 standard require the proper means of containment for this infectious substance
- Part 6 requires anyone who handles, offers for transport or transports the infectious substances to be properly trained and hold a training certificate in accordance with this part
Note: The transport of deceased bodies contaminated with the Ebola virus is not regulated under the TDG Regulations.
Shipping infectious substances
(PDF, 976 KB)








